Cell Ranger7.0, printed on 04/10/2025
| New in Cell Ranger v7.0: Intronic reads are counted by default for whole transcriptome gene expression data. For more information, see our recommendation on including introns for gene expression analysis page |
The 5' Chromium Next GEM Single Cell Immune Profiling Solution with Feature Barcode technology enables simultaneous profiling of V(D)J repertoire, cell surface protein, antigen specificity, and gene expression (GEX) data. The cellranger multi pipeline analyzes these multiple library types together, enabling more consistent cell calling between the V(D)J and gene expression data.
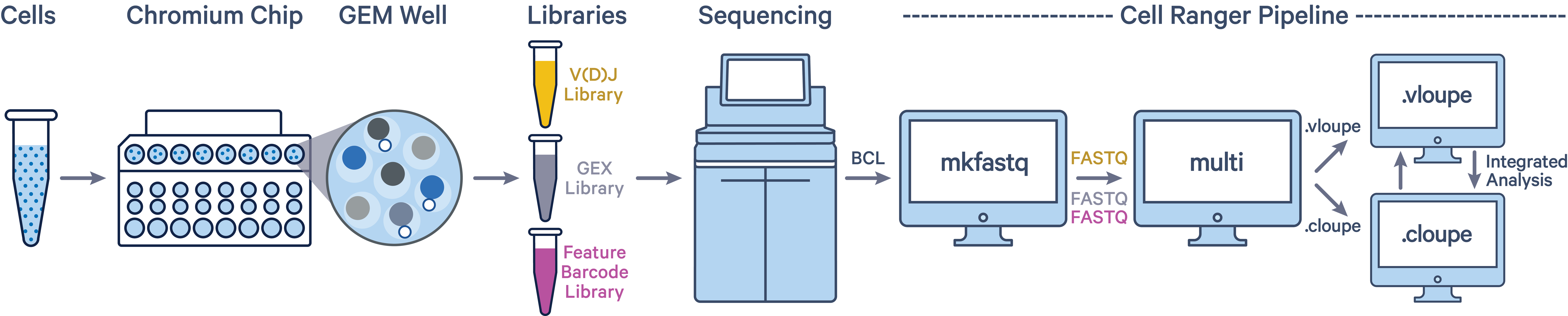
The cellranger multi pipeline takes a config CSV with paths to FASTQ files from cellranger mkfastq, bcl2fastq, or BCL Convert for any combination of 5' Gene Expression, Feature Barcode (cell surface protein, antibody/antigen, or CRISPR), and V(D)J libraries from a single GEM well. It performs alignment, filtering, barcode counting, and UMI counting on the Gene Expression and/or Feature Barcode libraries. It also performs sequence assembly and paired clonotype calling on the V(D)J libraries. Additionally, the cell calls provided by the gene expression data are used to improve the cell calls from the V(D)J data. Visit the multi tutorial page for self-guided and video tutorials on running cellranger multi.
Pipeline recommendation depends on the combination of libraries being analyzed. This table summarizes all library combinations and corresponding pipeline recommendations:
| Use multi? | |||
|---|---|---|---|
| Recommended. Improves final cell calls in V(D)J library. Learn more. | |||
| Recommended. Improves final cell calls in V(D)J library. Learn more. | |||
| Optional. Alternative pipeline: count + vdj | |||
| Optional. Alternative pipeline: vdj | |||
| Optional. Alternative pipeline: count | |||
| Optional. Alternative pipeline: count | |||
| Optional. Alternative pipeline: count |
The cellranger multi pipeline improves cell calls in the V(D)J dataset by discarding any cells that were not also called in the corresponding 5' gene expression dataset. By assigning cells that are called in the V(D)J results but not in the 5' gene expression results as background GEMs in the V(D)J data, the cellranger multi pipeline mitigates any overcalling issues that may arise in BCR and TCR data. This improved cell calling is only possible when both 5' Gene Expression and V(D)J libraries were sequenced from the same sample.
As shown in the image below, final V(D)J cell calls (intersection area) exclude cells that were only called by the vdj pipeline (yellow region).
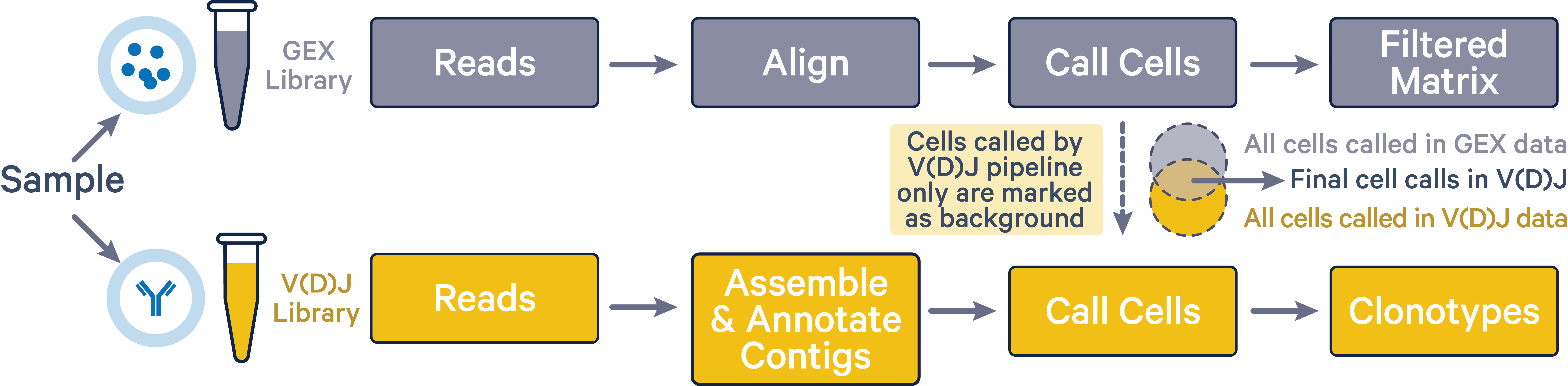
The 5' gene expression cell calls are not affected by the cellranger multi pipeline. The Gene Expression library is representative of the entire pool of poly-adenylated mRNA transcripts captured within each GEM. The TCR or BCR transcripts in the Gene Expression library are then selectively amplified to create the V(D)J library. Therefore, the Gene Expression library has more power to detect GEMs containing cells compared to the V(D)J library. If the cellranger multi pipeline is run with both 5' gene expression and V(D)J data, barcodes which are not called as cells in the 5' gene expression data are deleted from the V(D)J cell set.
The cellranger multi pipeline takes a config CSV file as input. The config CSV contains paths to FASTQ files for any combination of V(D)J, Gene Expression, and/or Feature Barcode libraries. To generate FASTQ files, follow the instructions for running cellranger mkfastq.
To simultaneously generate single cell feature counts, V(D)J sequences and annotations for a single library, run cellranger multi with the following arguments:
| Argument | Description |
|---|---|
--id | A unique run ID string: e.g. sample345 that is also the output folder name. Cannot be more than 64 characters. |
--csv | Path to multi config CSV file enumerating input libraries and analysis parameters. |
The multi config CSV contains both the library definitions and experiment configuration variables. It is composed of up to four sections: [gene-expression], [feature], [vdj], and [libraries]. The [gene-expression], [feature], and [vdj] sections have at most two columns, and are responsible for configuring their respective portions of the experiment. The [libraries] section specifies where input FASTQ files may be found. A customizable template for a multi config CSV can be downloaded here, and example multi config CSVs can be downloaded from public datasets. Example formats for a few product configurations are below.
Starting in Cell Ranger 7.0, the expected number of cells can either be auto-estimated or specified with expect-cells (e.g., to replicate a previous analysis), see Gene Expression algorithm overview. If needed, automated cell calling can be overridden with the force-cells option.
|
| Multi Config CSV | |
|---|---|
Section: [gene-expression] | |
| Field | Description |
reference | Path of folder containing 10x Genomics-compatible reference. Required for Gene Expression and Feature Barcode libraries. |
target-panel | Optional. Path to a target panel CSV file or name of a 10x Genomics fixed gene panel (pathway, pan-cancer, immunology, neuroscience). |
no-target-umi-filter | Optional. Disable targeted UMI filtering stage. Default: false. |
r1-length | Optional. Limit the length of the input Read 1 sequence of Gene Expression libraries to the first N bases, where N is a user-supplied value. Note that the length includes the Barcode and UMI sequences so do not set this below 26. This and r2-length are useful options for determining the optimal read length for sequencing. Default: do not trim Read 1. |
r2-length | Optional. Limit the length of the input Read 2 sequence of Gene Expression libraries to the first N bases, where N is a user-supplied value. Trimming occurs before sequencing metrics are computed and therefore, limiting the length of Read 2 may affect Q30 scores. Default: do not trim Read 2. |
chemistry | Optional. Assay configuration. NOTE: by default, the assay configuration is detected automatically, which is the recommended mode. Users usually will not need to specify a chemistry. Options are: auto for autodetection, fiveprime for Single Cell 5', SC5P-PE for paired end or SC5P-R2 for R2-only, SC-FB for Single Cell Antibody-only. Default: auto. |
expect-cells | Optional. Override the pipeline’s auto-estimate. See cell calling algorithm overview for details on how this parameter is used. If used, enter the expected number of recovered cells. |
force-cells | Optional. Force pipeline to use this number of cells, bypassing cell-calling algorithm. |
include-introns | Optional. Set to false to exclude intronic reads in count. Including introns in analysis is recommended to maximize sensitivity, except when target-panel is used. Default: true |
no-secondary | Optional. Disable secondary analysis, e.g. clustering. Default: false. |
no-bam | Optional. Set this flag to true to skip BAM file generation. This will reduce the total computation time for the pipestance and the size of the output directory. If unsure, we recommend not using this option, as BAM files can be useful for troubleshooting and downstream analysis. Default: false |
check-library-compatibility
| Optional. Allows users to disable the check that evaluates 10x Barcode overlap between libraries when multiple libraries are specified (e.g., Gene Expression + Antibody Capture). Setting this option to false will disable the check across all library combinations. We recommend running this check (default), however if the pipeline errors out, users can bypass the check to generate outputs for troubleshooting. Default: true |
Section: [feature] | |
| Field | Description |
reference | Optional. Feature reference CSV file, declaring Feature Barcode constructs and associated barcodes. Required only if Feature Barcode libraries are present. |
r1-length | Optional. Limit the length of the input Read 1 sequence of Feature Barcode libraries to the first N bases, where N is a user-supplied value. Note that the length includes the Barcode and UMI sequences so do not set this below 26. This and r2-length are useful options for determining the optimal read length for sequencing. Default: do not trim Read 1. |
r2-length | Optional. Limit the length of the input Read 2 sequence of Feature Barcode libraries to the first N bases, where N is a user-supplied value. Trimming occurs before sequencing metrics are computed and therefore, limiting the length of Read 2 may affect Q30 scores. Default: do not trim Read 2. |
Section: [vdj] | |
| Field | Description |
reference | Path of folder containing 10x Genomics-compatible V(D)J reference. Required for V(D)J Immune Profiling libraries. |
inner-enrichment-primers | Optional. If inner enrichment primers other than those provided in the 10x Genomics kits are used, they need to be specified here as a text file with one primer per line. |
r1-length | Optional. Limit the length of the input Read 1 sequence of V(D)J libraries to the first N bases, where N is a user-supplied value. Note that the length includes the Barcode and UMI sequences so do not set this below 26. This and r2-length are useful options for determining the optimal read length for sequencing. Default: do not trim Read 1. |
r2-length | Optional. Limit the length of the input Read 2 sequence of V(D)J libraries to the first N bases, where N is a user-supplied value. Trimming occurs before sequencing metrics are computed and therefore, limiting the length of Read 2 may affect Q30 scores. Default: do not trim Read 2. |
Section: [libraries] | |
| Column | Description |
fastq_id | Required. The Illumina sample name to analyze. This will be as specified in the sample sheet supplied to mkfastq or bcl2fastq. |
fastqs | Required. The folder containing the FASTQ files to be analyzed. Generally, this will be the fastq_path folder generated by cellranger mkfastq. |
lanes | Optional. The lanes associated with this sample, separated by |. Defaults to using all lanes. |
feature_types | Required. The underlying feature type of the library, which must be one of Gene Expression, VDJ, VDJ-T, VDJ-T-GD, VDJ-B, Antibody Capture, or CRISPR Guide Capture. Setting to VDJ will auto-detect the chain type. Auto-detection of chain type only works when all V(D)J FASTQs are the same chain type (i.e. auto-detection with feature_type set to VDJ fails if users have both TCR and BCR FASTQ sets). Auto-detection does not work for TRG/D (gamma-delta) chains. If set to auto-detection, gamma-delta libraries are treated as TCR and gamma-delta chains are filtered out. The pipeline runs to completion, but zero barcodes are assigned to cells. Auto-detection only works for TCR libraries with alpha-beta chains and BCR libraries. For TRG/D chains, set feature_type to VDJ-T-GD. If the chain for one V(D)J FASTQ set is specified, chains for all existing V(D)J FASTQ sets must be specified. Valid specifications include: VDJ, VDJ-T, VDJ-B, or VDJ-T-GD, and the combinations VDJ-T & VDJ-B and VDJ-T-GD & VDJ-B and VDJ-T & VDJ-T-GD & VDJ-B. Note that gamma-delta analysis is enabled but the algorithm has not been tested extensively. |
subsample_rate | Optional. The rate at which reads from the provided FASTQ files are sampled. Must be strictly greater than 0 and less than or equal to 1. |
For help on how to configure the [libraries] section to target a particular set of FASTQs, consult Specifying Input FASTQ Files for cellranger multi.
|
After determining the input arguments, run cellranger multi. Remember to replace the bits of code in red with your sample id and csv file path:
mkdir /home/jdoe/runs cd /home/jdoe/runs cellranger multi --id=sample345 --csv=/home/jdoe/sample345.csv
Following a series of checks to validate input arguments, cellranger multi pipeline stages will begin to run:
Martian Runtime - v4.0.8 Running preflight checks (please wait)... ...
By default, cellranger will use all of the cores available on your
system to execute pipeline stages. You can specify a different number of cores
to use with the --localcores option; for example, --localcores=16
will limit cellranger to using up to sixteen cores at once. Similarly,
--localmem will restrict the amount of memory (in GB) used by
cellranger.
The pipeline will create a new folder named with the run ID you specified using the --id argument (e.g. /home/jdoe/runs/sample345) for its output. If this folder already exists, cellranger will assume it is an existing pipestance and attempt to resume running it. If you wish to start the run over, delete the output folder (sample345/ in this example) and rerun the pipeline.
A successful cellranger multi run should conclude with a message similar to this:
Waiting 6 seconds for UI to do final refresh. Pipestance completed successfully! yyyy-mm-dd hh:mm:ss Shutting down. Saving pipestance info to "tiny/tiny.mri.tgz"
To learn more about the output files generated, refer to the Outputs for multi section under Understanding Outputs.
The cellranger multi pipeline allows users to analyze T cell libraries enriched for gamma (TRG) and delta (TRD) chains. 10x Genomics does not provide reagents or primers for TRG/D chain enrichment. Since this workflow is not fully supported, the Cell Ranger pipeline has not be extensively tested for TRG/D libraries, and the algorithm's performance cannot be guaranteed.
To analyze TRG/D libraries, set feature_types to VDJ-T-GD in the [libraries] section of the multi config CSV. Auto-detection does not work for TRG/D chains. If set to auto-detection, TRG/D libraries are treated as TCR libraries enriched for alpha-beta chains, and the gamma-delta chains are filtered out. The pipeline runs to completion, but zero barcodes are assigned to cells.
Refer to the example multi config CSV for additional configuration guidance. Outputs from a successful gamma-delta run are located in the vdj_t_gd folder.
The cellranger vdj pipeline cannot process FASTQs from TRG/D enriched libraries.
Here are the example multi config CSVs for a few commonly used library combinations. Make sure to replace /path/to with the actual full path to your data, and edit any text in red according to the experiment's sample/library/file names. For TRG/D specific examples, click here.
| Libraries | Multi config CSV |
 See example dataset |
[vdj] reference,/path/to/vdj_reference |
 See example dataset |
[gene-expression] reference,/path/to/transcriptome |
 See example dataset |
[gene-expression] reference,/path/to/transcriptomeThis template also applies to V(D)J + FB (without GEX) libraries. The [gene-expression] reference section is required. However, the GEX FASTQ specification under the [libraries] section must be removed for the VDJ+FB library combinations.
|
The cellranger multi pipeline supports downsampling the reads by specifying a rate between 0 and 1 independently for each library. It also allows trimming the reads to a fixed length, which is not supported in the cellranger vdj pipeline.
The option to run denovo without V(D)J reference (--denovo) is not supported in cellranger multi. This option is available in cellranger vdj.
Next, you may wish to: